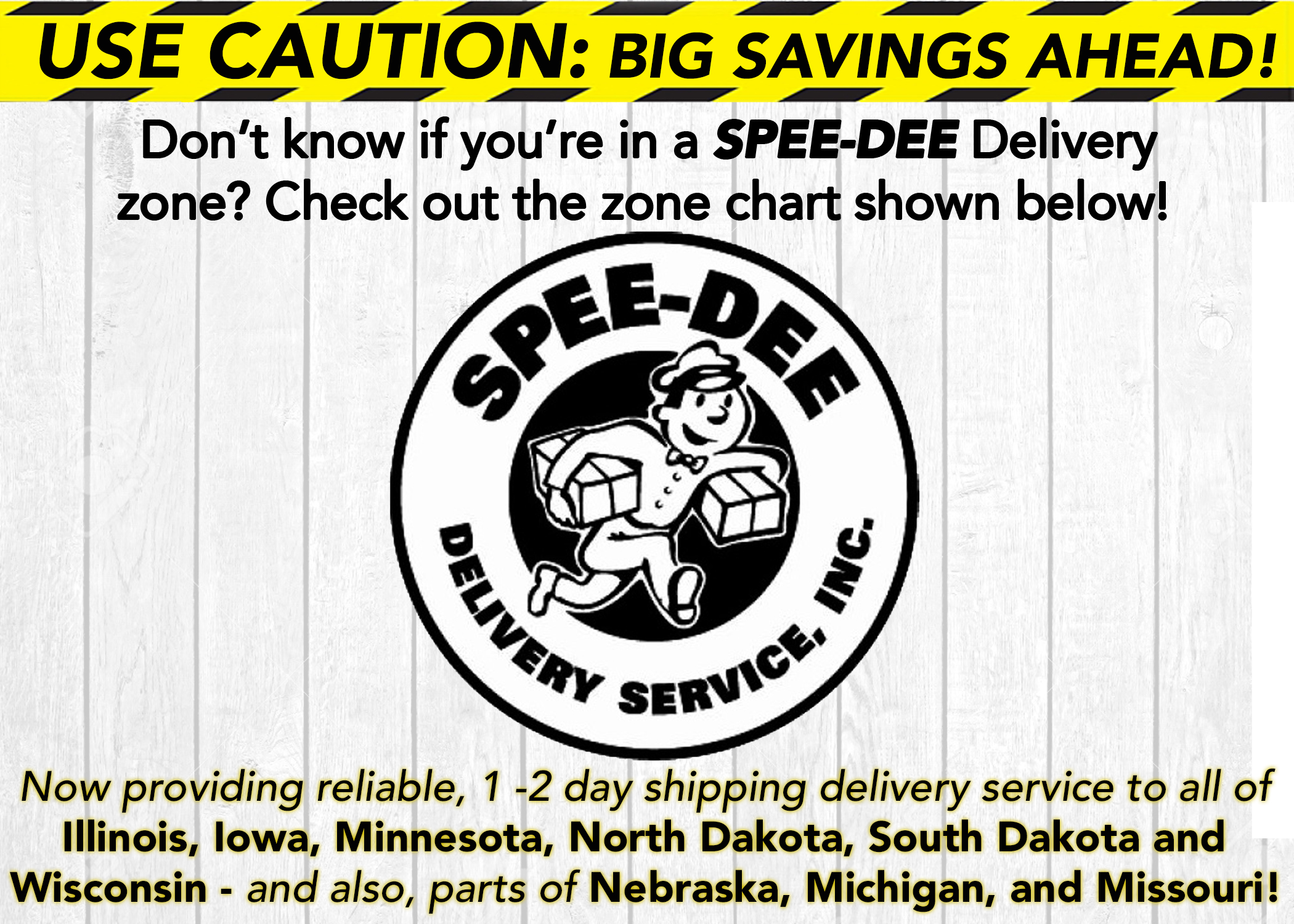
MUSCLE CAR MANIA: Chevy* Power
We look under the hood at classic Chevy muscle car engines and the products to protect them.

 _by Brad Nelson|March 1, 2024
_by Brad Nelson|March 1, 2024
The glory days of the muscle-car era were fueled by a war between American automakers for stoplight-to-stoplight power and speed. The victors were speed demons who craved increasingly powerful engines that were stuffed into sleek small and midsized sedans. These large-displacement engines offered thunderous excitement with rubber-shredding horsepower. Eventually, stricter emissions, oil embargoes and skyrocketing insurance premiums brought the golden age of American muscle to an end, but legends never die. In this edition of Muscle Car Mania, we delve into a few of the mythical Chevrolet* muscle-car engines that were too good to forget.
SMALL BLOCK
In the early 1950s, the hot-rod community shrugged Chevy off with its reliable, but underwhelming, Stovebolt Six* engines. But everything changed in the fall of 1954 with the launch of the groundbreaking small-block V8. Once speed enthusiasts discovered this lightweight, compact powerhouse, it outshined the flathead Ford* as the star of the strip. The first-generation small-block Chevy V8 has had an impact like no other eight-cylinder engine in history due to its simplicity and compact power. These engines were easy to work on, with opportunities to upgrade components. The first-generation small blocks offered variants that approached 400 horsepower by the early 1970s. Affordable and easy to find, the original small block remains the most popular high-performance classic-car engine in the world.

265 V8
In 1955 and ’56, the 265 small-block V8 powered over half of all new Chevys. The engine came in three configurations: the 162-hp two-barrel, the 180-hp Power Pack* with four-barrel and dual exhaust and the coveted 195-hp Super Power Pack* with a solid-lifter Duntov* cam, higher-compression pistons and free-flowing dual exhaust. Over the next couple years, the 265 added dual four barrels and fuel injection to put out 283 horses in 1957, 327 hp in 1962 and 350 hp in 1966. Horsepower ratings reached up to 375 in the Corvette.* In all, over 1.5 million 265-powered Chevrolets were sold.
283 V8
The 238 V8 powered vehicles from 1957 to 1967. It was incredibly versatile, but classic-car enthusiasts remember it as the first production engine that could produce one horsepower per cubic inch of displacement using a Duntov camshaft and Ramjet* fuel injection. Enthusiasts upped the ante by boring the cylinder walls for up to 301 cubes. In the ’60s, enthusiasts started adding larger intake valve heads and dual carbs, or an aluminum high-rise four-barrel Carter* AFB or Holley* intake.

L65 327 V8
From 1958 through 1964, Chevy bored and stroked the 283 to 327 cubic inches. The highest factory rating for the 327 in 1964 and ’65 was 375 hp in Corvettes with Ramjet fuel injection. The power curve was 2,700 to 7,200 rpm. Some 327s were equipped with a new 750- cfm, dual-inlet Holley 3310 carb for even more power.
348 V8
The 348 V8 was originally designed for heavy-duty trucks, but to enhance performance, Chevy added more
compression, a high-lift camshaft and tri-power induction. The production model was a torque beast capable of making over 300 horsepower to about 5,500 rpm. The 348 frequently put Chevy in the winners circle in 1960 and ’61.
409 V8
“Giddy up, giddy up, 409,” sang the Beach Boys in their hit song “409” about a “four-speed, dual-quad, posi-traction 409.” In 1961, the famous 348 was taken to another level with a high-performance variant known as the 409, a bored and stroked 348 with larger head ports and valves. Despite heavy pistons, the 409 was the engine to beat in everything except NASCAR* races, where the weighty pistons hammered away at reliability. But almost all top professional drag racers ran and won with a 409 in 1962 and ’63.
L78 396
In 1965, two Turbo Jet* 396 big-block engines replaced the 409, one of which was the factory-rated 425 hp RPO L78, a high-performance engine with rectangle-port heads, 11.0:1 compression and an aluminum high-rise intake manifold with an 800 cfm Holley carb. The L78 was put into Corvettes for an extra cost of $292.70. At the time, the L78 396 provided the highest acceleration and top speed of any production engine Chevrolet ever produced.
L72 427
The L72 427 V8 was first put into 1966 Corvettes, and later into the massive full-size passenger cars of the era. The engine was marketed at 450 hp for 1966 models, but later reduced to 425 hp, ostensibly to reduce insurance rates for would-be owners. Regardless, the L72 427 was a winner on all fronts and became the foundation for all Chevrolet solid-lifter big-block engines through 1969. Muscle cars using the L72 include the Chevelle,* Nova* and Camaro.*

427 L88
The 1967-1969 production 427 L88 race engine was marketed at only 430 hp at 5,200 rpm, but at 7,400 rpm, the 12.5:1-compression, mega-cam, rectangle-port 427 could churn out 550 hp. Only available in the Corvette, this engine put out so much heat that it was very difficult to keep cool, but it could slay other engines in street races.
454 BIG-BLOCK V8
The Chevy 454 big-block V8 was the right engine at the wrong time. GM* introduced the 454 in 1970, one year before emission standards were tightened and three years before the gas crisis hit. It was unfortunate timing for the mighty V8 designed for performance cars, including the Chevelle and Corvette, but the 454 made an indelible mark nonetheless. With high compression, solid-lifter camshaft, huge valve lift and massive 800 cfm Holley carburetor, output was listed at 450 hp and 500 lb-ft of torque, which was more than enough to shred tires at the drop of a hat.
PROTECT YOUR CHEVY POWER
If you’re lucky enough to have your foot on the accelerator of a legendary Chevy V8, protection is priority. Here’s a list of AMSOIL products to help keep your classic muscle car ripping far into the future.
AMSOIL Assembly Lube

As they say, a great engine isn’t built in a day. Partially assembled engines can sit idle for weeks or months at a time. During this process, an engine-assembly lube must be applied that will cling to parts and provide wear protection, inhibit rust and help prevent deposit formation. AMSOIL Engine Assembly Lube handles all of the above.
AMSOIL Break-In Oil

Freshly rebuilt engines should start off with AMSOIL Break-In Oil. It’s formulated with zinc and phosphorus anti-wear additives to protect critical components during the break-in period when engine wear rates are highest. It doesn’t contain friction modifiers to allow for quick and efficient piston-ring seating, an important aspect of the break-in process to ensure maximum power and engine longevity.
AMSOIL Z-ROD® Synthetic Motor Oil

AMSOIL Z-ROD® is engineered specifically for classic and high-performance vehicles to perform on the street and protect during storage. It features a high-zinc formulation that protects flat-tappet camshafts and critical engine components, along with a proprietary blend of rust and corrosion inhibitors for added protection during long-term storage. It’s available in 10W-30, 10W-40 and 20W-50 viscosities.
AMSOIL Miracle Wash® Waterless Wash and Wax Spray

AMSOIL Miracle Wash is a must-have for owners dedicated to keeping their vehicle’s appearance on par with its performance. Simply spray and wipe off to lift dirt away from the surface instantly. It leaves vehicles with a super-shiny finish that protects against dust, light dirt and harmful ultraviolet rays.
AMSOIL DOMINATOR® Octane Boost

Early V8 models were designed to use leaded gasoline. As a result, classic and collector autos often require the use of a lead substitute to preserve the components that were designed for the fuel of days gone by. AMSOIL DOMINATOR Octane Boost is excellent as a lead substitute in older vehicles. It increases octane up to four points, helping reduce engine knock and improving ignition while helping fuel burn more cleanly.
AMSOIL Gasoline Stabilizer

When it’s time to put her away at the end of the season, AMSOIL Gasoline Stabilizer is crucial to ensuring your ride is road-ready in spring. Gasoline can degrade in as few as 30 days. Treat your fuel tank prior to parking the vehicle for the winter to help prevent fuel degradation and poor engine performance when it’s time to fire it back up.
AMSOIL Engine Fogging Oil

Any engine facing storage or lengthy inactivity should be treated with a good dose of AMSOIL Engine Fogging Oil first. Giving the cylinders a shot of oil protects them from rust, corrosion and harmful dry starts when it comes time to fire up your hot rod or classic car the following season.

*All trademarked names and images are the property of their respective owners and may be registered marks in some countries. No affiliation or endorsement claim, express or implied, is made by their use.








 _by
_by 



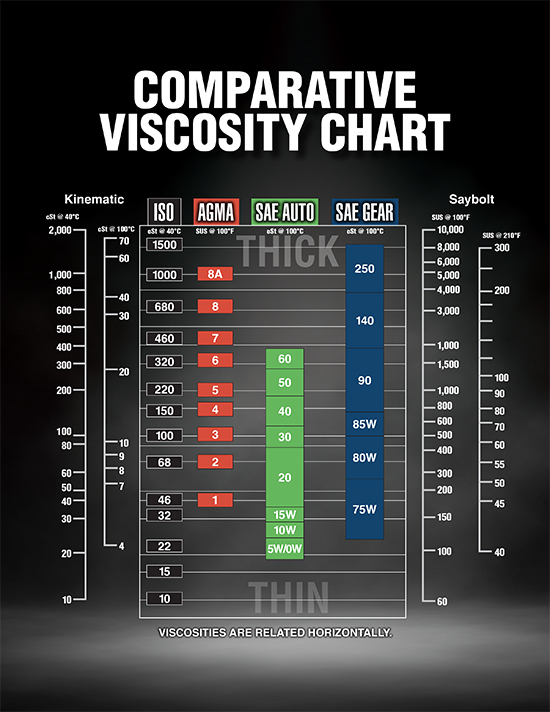





 _by David Hilgendorf | September 29, 2023
_by David Hilgendorf | September 29, 2023



